Abstract
Introduction: Systemic mastocytosis (SM) is a rare myeloid neoplasm driven by the KIT D816V mutation in ~95% of patients. Patients with advanced SM (AdvSM) have a poor prognosis and treatment options are limited. Avapritinib, an oral, potent, highly selective inhibitor of KIT D816V, is approved in the USA for treatment of adults with AdvSM, and in Europe after ≥1 prior systemic therapy. Avapritinib is not recommended for the treatment of patients with AdvSM with platelet counts <50×109/L. Data from the multi-center, international, phase 1 EXPLORER (NCT02561988) and phase 2 PATHFINDER (NCT03580655) clinical studies demonstrated that responses to avapritinib treatment were rapid, deep, and durable regardless of prior therapy, AdvSM subtype, or presence of high-risk mutations. Here we present analyses from patients with AdvSM treated with avapritinib as first-line therapy in PATHFINDER.
Methods: Analyses included adult patients with centrally confirmed AdvSM without prior antineoplastic treatment for AdvSM (treatment-naïve) who initiated 200 mg once-daily avapritinib in the PATHFINDER study. The primary endpoint was overall response rate (ORR), centrally evaluated according to the modified International Working Group-Myeloproliferative Neoplasms Research and Treatment-European Competence Network on Mastocytosis (mIWG-MRT-ECNM) response criteria defined as complete remission with full (CR) or partial (CRh) recovery of peripheral blood counts, partial remission (PR), or clinical improvement (CI). Secondary endpoints included, but were not limited to, mean change from baseline measures of disease burden (bone marrow mast cell [BM MC] burden, serum tryptase, blood KIT D816V variant allele fraction [VAF], and spleen volume), duration of response (DOR), time to response (TTR), overall survival (OS) and safety.
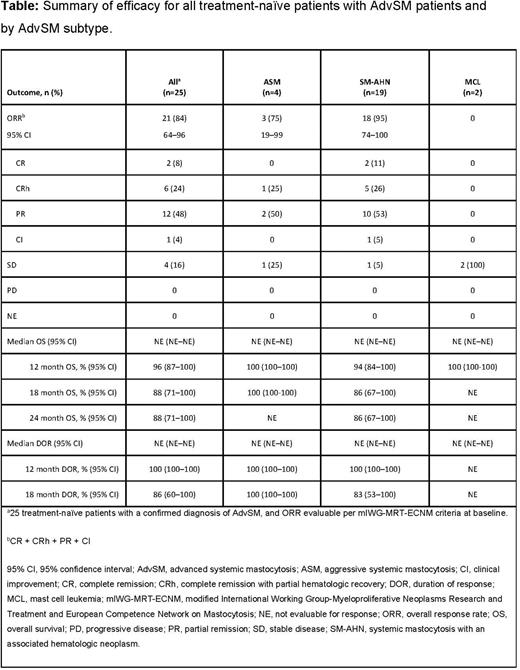
Results: As of April 20, 2021, 107 patients with centrally confirmed AdvSM were enrolled, of whom 38 were treatment-naïve; 18% (n=7/38) had ASM, 74% (n=28/38) SM-AHN, and 8% (n=3/38) MCL. Median age (range) was 68 years (39-88), 53% were male, 16% had Eastern Cooperative Oncology Group performance status 2-3, and 95% exhibited KIT D816V. In 25 of 38 (66%) mIWG-MRT-ECNM response-evaluable patients, the ORR (95% CI) was 84% (n=21/25; 64-96) with 32% (n=8/25) achieving CR or CRh, 48% (n=12/25) achieving PR, and 4% (n=1/25) CI (Table). The median TTR (range) was 2.0 months (0.3-12.2). Median DOR across all subtypes was not reached at the time of analysis; however, the DOR rate (95% CI) was 100% (100-100) at 12 months and 86% (60-100) at 18 months. For patients with ASM, the DOR rate was 100% (100-100) at 12 and 18 months. In patients with SM-AHN, the DOR rate was 100% (100-100) at 12 months and 83.3% (53.5-100) at 18 months. After a median follow-up of 9.7 months, median OS in all patients was not reached. The OS rate was 96% (95% CI: 87-100) at 12 months and 88% (95% CI: 71-100) at 24 months. The OS rate at 12 months was 100% for patients with ASM or MCL and 94% (84-100) for patients with SM-AHN. At 24 months, OS rate was 86% (67-100) for patients with SM-AHN. In almost all patients, reductions of ≥50% in measures of disease burden including reduction in BM MC burden (84%, n=32/38), serum tryptase (95%, n=36/38), KIT D816V VAF (89%, n=34/38), and ≥35% reduction in spleen volume (66%, n=25/38) were observed. Total clearance of neoplastic MC aggregates in BM was achieved in 63% of patients (n=24/38). The most frequent (≥25% of N=38) treatment-related adverse events (TRAEs) were (any grade, grade ≥3) periorbital edema (53%, 5%), thrombocytopenia (45%, 21%), peripheral edema (37%, 3%), and anemia (26%, 13%). In total, 7 (18%) patients experienced cognitive effects, all grade 1 or 2; no intracranial bleeding events and no treatment-related deaths were reported. 28 (74%) patients had dose reductions and 25 (66%) had dose interruptions due to TRAEs. Thrombocytopenia was the most common TRAE leading to dose reduction (21%, n=8/38) and dose interruption (26%, n=10/38). 4 (10%) patients discontinued treatment due to TRAEs; 1 discontinuation each was due to neutropenia, thrombocytopenia, cognitive effects, and worsening weight loss.
Conclusions: Avapritinib demonstrated a high level of efficacy as the first-line therapy for patients with AdvSM across all disease subtypes with an ORR of 84% and an OS rate of 88% at 2 years. Avapritinib treatment with a 200 mg once-daily starting dose was generally well-tolerated.
Disclosures
Radia:Cogent Biosciences: Other: Steering committee member for APEX study; Blueprint Medicines Corporation: Membership on an entity's Board of Directors or advisory committees, Other: Educational events, Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees, Other: Educational events. Gotlib:Blueprint Medicines Corporation: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Cogent Biosciences: Consultancy, Research Funding; Incyte: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Deciphera: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Research Funding; Kartos: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; PharmaEssentia: Honoraria, Membership on an entity's Board of Directors or advisory committees; Allakos: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Drummond:Blueprint Medicines Corporation: Research Funding; Novartis: Other: Personal fees, Research Funding. George:Celgene/BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees; Blueprint Medicines Corporation: Consultancy, Membership on an entity's Board of Directors or advisory committees; ARUP Laboratories: Other: Associated; Cogent Biosciences: Consultancy, Membership on an entity's Board of Directors or advisory committees. Lin:Blueprint Medicines Corporation: Current Employment, Current equity holder in publicly-traded company. Dimitrijević:Blueprint Medicines (Switzerland) GmbH: Current Employment, Current equity holder in publicly-traded company. Munoz-Gonzalez:Blueprint Medicines (Switzerland) GmbH: Current Employment, Current equity holder in publicly-traded company. Bidollari:Blueprint Medicines Corporation: Current Employment, Current equity holder in publicly-traded company. Deininger:Novartis: Consultancy, Honoraria, Research Funding; Incyte: Consultancy, Honoraria, Research Funding; Blueprint Medicines Corporation: Consultancy, Honoraria, Research Funding; Pfizer Inc: Consultancy, Honoraria, Research Funding; Galena Biopharma: Consultancy, Honoraria; Bristol Myers Squibb: Consultancy, Honoraria, Research Funding. DeAngelo:Incyte: Consultancy; Gilead: Consultancy; Blueprint Medicines Corporation: Consultancy, Research Funding; Forty-Seven: Consultancy; AbbVie: Research Funding; Pfizer: Consultancy; Novartis: Consultancy, Research Funding; Kite, a Gilead Company: Consultancy; Takeda: Consultancy; Glycomimetics: Research Funding; Shire: Consultancy; Jazz: Consultancy; Autolos: Consultancy; Agios: Consultancy; Servier: Consultancy; Amgen: Consultancy. Reiter:Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AOP: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; GSK: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Blueprint Medicines: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal